BLOOD AND DISEASES
SUMMARY PRESENTATION OF BLOOD COMPONENTS / AUTOTRANSFUSION
THE STORY OF BLOOD IN THE WORLD
THE STORY OF BLOOD IN BRAZIL
Are we going to change, take reliable information and be part of this change in history? AUTOTRANSFUSION
What is blood in reality?
We know that; "Blood is a special type of connective tissue that stands out for presenting itself as a red and viscous fluid. It is characterized by having a liquid matrix (plasma), in which the cellular elements of the blood (red blood cells) are suspended. , leukocytes and platelets)."
However, it is more related to RISK than benefit, the doctor needs to give the patient the best treatment and blood is not the best, according to more recent research there are better alternatives. because according to experts the blood transfusion brings serious problems to the patient. It sounds simple but it's not. Cellular injuries, immunobiological diseases and the presence of viruses and bacteria. There are flaws and complications.
Let's say, my blood is excellent, it knows me, it knows my organs, it protects me. He defends any aggressor, any foreign body that comes to my body, he knows how to defend himself.
Now another blood in you is a disaster even if there was compatibility someone else's organs are different from my organs, with the transfusion your own blood will fight itself. Antigens fighting antibodies, your body will also reject donated blood because it's not yours either.
Stored blood receives both biomechanical and biophysical changes.
The blood stored in the refrigerator undergoes a globular change. Its shape is usually of a biconcave disk, when stored in the refrigerator it transforms like a castor bean, full of spikes, the blood also hardens (8.0 micrometers) and causes difficulty for the passage, so it does not pass through the capillary (4.4 micrometers) ) obstructing the microcirculation. The patient who uses blood is related to death and kidney failure. Deleterious effects on storage, handling, safety itself. It's best not to use someone else's blood. Stay healthy, use AUTOTRANSFUSION.
Technologies can solve these problems. ABA helps you.
Understand more...
Components
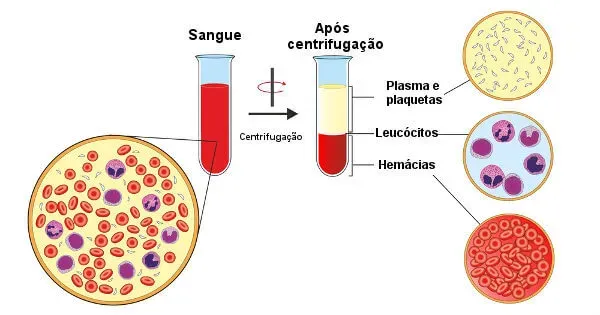
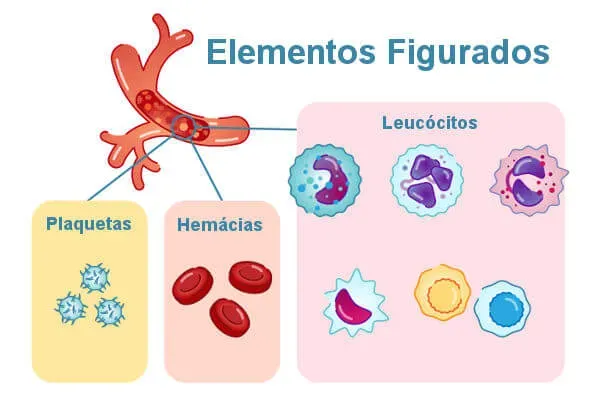
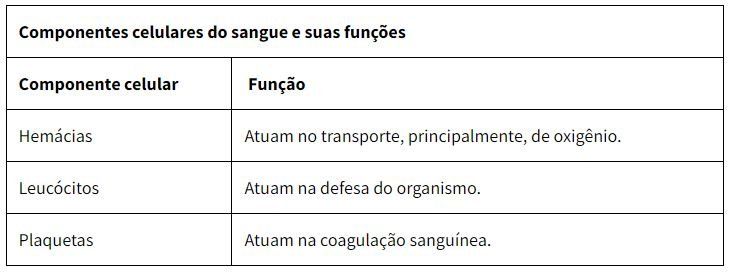
Blood is composed of blood plasma, two cell types (erythrocytes and leukocytes) and cell fragments called platelets. Erythrocytes, leukocytes and platelets are called formed elements of blood. These elements constitute 45% of the blood volume, while plasma constitutes 55% of its volume.
A healthy person has a total blood volume of approximately 7% of their body weight.New Paragraph
Blood plasma
Blood plasma is the liquid part of blood and is pale yellow in color. This represents more than half of the total volume of blood in our body and is 90% made up of water.
In the plasma are still found mineral salts, proteins, hormones, among other substances, as nutrients and residues of the metabolism. It is in the plasma that the figurative elements are suspended.
RBCs, red blood cells, or erythrocytes
Red blood cells, also known as red blood cells and erythrocytes, are the cells responsible for transporting oxygen in the body. They are anucleate cells, that is, they do not have a nucleus and are shaped like a biconcave disk. These cells are small, about seven to eight micrometers in diameter.
Red blood cells have a short life span, which lasts about 120 days, and are later destroyed, mainly in the spleen. Under normal conditions, these cells do not leave the interior of blood vessels.
The red color of these cells is due to the presence of a protein called hemoglobin, which, in addition to ensuring color, is responsible for transporting oxygen in the body.
The absence of a nucleus in red blood cells favors the increase of space for hemoglobin in these cells. It is worth noting that, in addition to the absence of a nucleus, red blood cells also lack mitochondria.
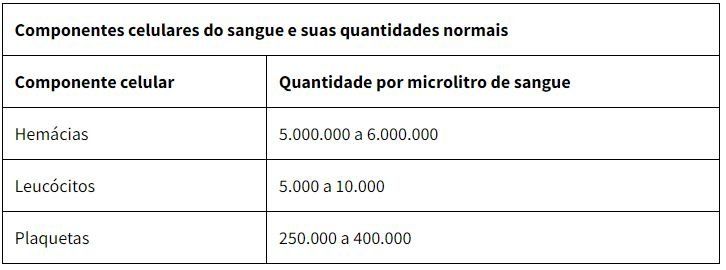
When we observe a reduction in the number of red blood cells in the blood, we have a condition known as anemia. The blood is red due to the large amount of red blood cells found in it. This cell is found in greater quantity, with about five to six million erythrocytes being observed in each microliter of blood.
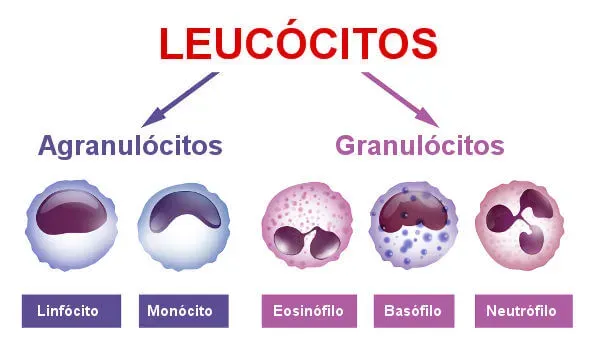
Leukocytes or white blood cells
Leukocytes, also called white blood cells, are the cells responsible for the defense of our body. They are colorless, have a spherical shape and are capable of performing diapedesis, which is their active exit from the blood vessels, to act in the defense function in injured tissues or attacked by pathogenic agents. On average, every microliter of blood contains five to ten thousand leukocytes.
There are different types of leukocytes, each performing a specific function related to the protection of the body. Some of them, for example, carry out the process of phagocytosis, others are responsible for the production of antibodies, which are defense proteins. Neutrophils, basophils, monocytes, eosinophils, and lymphocytes are types of leukocytes.
Blood
Blood is a special type of connective tissue related, among other functions, with the transport of nutrients and respiratory gases and the defense of the body.
Blood is a connective tissue with special properties.
Blood is a special type of connective tissue that stands out as a red, viscous fluid. It is characterized by having a liquid matrix (plasma), in which the cellular elements of the blood (red blood cells, leukocytes and platelets) are suspended.
In humans, blood flows within our cardiovascular system, which is closed off. This means that our blood is found only inside the heart and our blood vessels.
Generally, a person has a total blood volume that corresponds to about 7% of their body weight. With that, we have that an individual of approximately 70 kilos, must present about five liters of blood.
→ Function
Blood has several functions in the body, ensuring, for example:
- Transport of nutrients;
- Transport of respiratory gases;
- Transport of metabolic waste;
- Defense and immunity through the action of leukocytes;
- Blood clotting through the action of platelets.
On a blood count, increased leukocyte values may represent an infection.
Leukocytes are divided into two groups: granulocytes and agranulocytes.
Granulocytes are characterized by having an irregularly shaped nucleus and specific granules in their cytoplasm. Neutrophils, eosinophils, and basophils are granulocyte-type leukocytes.
Agranulocytes, unlike the previously mentioned group, have a more regular shaped nucleus and the presence of specific granules is not observed in their cytoplasm. Lymphocytes and monocytes are examples of agranulocytes.
platelets
Platelets are fragments of bone marrow megakaryocytes, that is, they are not cells themselves. These structures are about two to three micrometers in diameter and also lack a core.
They act in the clotting process and also help in the repair of blood vessels that have suffered some kind of injury. In every microliter of blood, there are about 150,000 to 450,000 platelets.
In patients with dengue, a drop in the number of platelets is observed. In classic dengue, it is observed that this count is below 100 thousand in each microliter of blood.
where it is produced
Blood is produced in the so-called bone marrow. This is located in the cavities of cancellous bones and also in the medullary canal of long bones.
TRANSFUSION DISEASES
The most common complications of transfusion are
- Febrile nonhemolytic reactionsReactions with chills
The most serious complications, with very high mortality rates, are
- Acute hemolytic reaction (RTHA) due to ABO incompatibility Graft-versus-host disease (GVHD)Transfusion-associated circulatory overloadTransfusion-related acute lung injury (LPART)
Other complications include
- Allergic reactionsAltered oxygen affinityDelayed hemolytic transfusion reactionInfectionsPost transfusional purpura
Early recognition of symptoms suggestive of a transfusion reaction and prompt information to the blood bank are essential. The most common symptoms are chills, stiffness, fever, dyspnea, dizziness, hives, itching, and flank pain. If any of these symptoms (other than hives and localized itching) occur, the transfusion should be stopped immediately and the IV line kept patent with normal saline. Rest of the blood product and anticoagulated and clotted samples of the patient's blood should be sent to the blood bank for investigation. NOTE: The unit in question must not be restarted and the transfusion of any previously provided unit must not be started. Additional transfusion should be delayed until the cause of the reaction is known, unless the need is urgent, in which case Rh negative type O red cells should be used.
Hemolysis of the donor or recipient (usually the first) during or after transfusion may result from ABO/Rh incompatibility, plasma antibodies, or fragile or hemolyzed erythrocytes (eg, overheating of stored blood or contact with hypotonic IV solutions) . Hemolysis is more common and severe when incompatible erythrocyte donors are hemolyzed by antibodies in the recipient's plasma. Hemolytic reactions can be acute (within 24 h) or late (1 to 14 days).
Febrile non-hemolytic transfusion reaction
Febrile reactions may occur without hemolysis. Antibodies directed against the human leukocyte antigen (HLA) of leukocytes in the blood of an otherwise compatible donor are a possible cause. This cause is the most common in multitransfused or multiparous patients. Cytokines released from leukocytes during storage, particularly in platelet concentrates, are another possible cause.
Clinically, febrile reactions consist of an increase in temperature ≥ 1°C, chills, and sometimes headache and back pain. Simultaneous symptoms of an allergic reaction are common. Since fever and chills also portend a severe hemolytic transfusion reaction, all febrile reactions should be investigated, as should all severe hemolytic transfusion reactions.
Most febrile reactions are successfully treated with acetaminophen and, if necessary, diphenhydramine. Patients should also be treated (eg, with paracetamol) before future transfusions. If the recipient has had more than one febrile reaction, special leukoreduction filters are used for future transfusions; many hospitals use pre-stored leukoreduced blood components.
Acute hemolytic transfusion reaction (ARTHA)
About 20 people die each year in the US from an acute hemolytic transfusion reaction. RTHA usually results from the reaction of antibodies in the recipient's plasma against the donor's erythrocytes. ABO antigen incompatibility is the most common cause of an acute hemolytic transfusion reaction. Antibodies to non-ABO blood group antigens can also cause RTHA. The usual error is the lack of identification (label) in the pre-transfusion sample of the recipient at the time of collection and the failure to cross the sample of the recipient in question with the blood component immediately before transfusion.
Hemolysis is intravascular, causing hemoglobinuria with varying degrees of failure, acute kidney injury and possibly disseminated intravascular coagulation (DIC). The severity of an acute hemolytic transfusion reaction depends on
- Degree of incompatibility Amount of blood administered Speed of administration Integrity of kidneys, liver and heart
The acute phase usually occurs within the 1st hour of initiation of the transfusion, but may occur later during the transfusion or immediately afterwards. The beginning is often abrupt. The patient may complain of discomfort and anxiety. There may be dyspnea, fever, chills, facial flushing, and severe pain, especially in the lower back. There may be evolution to shock, with a rapid and weak pulse; cold, clammy skin; low blood pressure; and nausea and vomiting. Jaundice may follow after acute hemolysis.
If RTHA occurs under general anaesthesia, the only symptoms may be hypotension, uncontrollable bleeding at incision sites and mucous membranes from associated disseminated intravascular coagulation (DIC), or dark urine reflecting hemoglobinuria.
If RTHA is suspected, one of the first steps is to recheck the specimen and patient IDs. The diagnosis is confirmed by a positive direct antiglobulin test, measurement of urinary hemoglobin, serum lactic dehydrogenase (LDH), bilirubin, and haptoglobin. Intravascular hemolysis produces free hemoglobin in plasma and urine; haptoglobin levels are very low. Subsequent hyperbilirubinemia may occur.
After the acute phase, the degree of acute kidney injury determines the prognosis. Diuresis and blood urea decrease usually require recovery. Permanent kidney failure is not common. Prolonged oliguria and shock are signs of poor prognosis.
If RTHA is suspected, the transfusion should be stopped and supportive care initiated. The goal of initial therapy is to adequately achieve and maintain arterial blood pressure and renal blood flow with 0.9% IV saline and furosemide. IV saline is given to maintain urine output at 100 mL/h for 24 hours. The initial dose of furosemide is 40 to 80 mg (1 to 2 mg/kg in children), with future doses adjusted to maintain urinary flow > 100 mL/h on the first day.
Drug treatment of hypotension should be done with caution. Blood pressure drugs that decrease renal blood flow (eg, adrenaline, noradrenaline, high-dose dopamine) are contraindicated. If pressure medication is needed, usually dopamine, 2 to 5 mcg/kg/min, is used.
The nephrologist should be consulted as soon as possible, particularly if there is no diuretic response within 2 to 3 h of initiation of therapy, which may indicate acute tubular necrosis. Additional fluid and diuretic therapy may be contraindicated and early dialysis may be helpful.
Graft-versus-host disease (GVHD)
The cause of graft-versus-host disease (see also Graft-versus-host disease and graft-versus-host disease) associated with transfusions is usually caused by the transfusion of immunocompetent lymphocyte-containing products into an immunocompromised host. The donor's lymphocytes attack the host's tissues because their immune system cannot destroy the donor's lymphocytes. Graft versus host disease can occasionally occur in immunocompetent patients if they receive blood from a donor (often a close relative) who is homozygous for a human leukocyte antigen (HLA) haplotype for which the patient is heterozygous. Signs and symptoms include fever, rash (skin rash that spreads centrifugally, becoming blistered erythroderma), vomiting, watery, bloody diarrhea, lymphadenopathy, and pancytopenia due to bone marrow aplasia. Jaundice and elevated levels of liver enzymes are also common. Graft versus host disease occurs 4 to 30 days after transfusion and is diagnosed based on clinical suspicion and skin and bone marrow biopsies. GVHD has >90% mortality, as there is no specific treatment available.
The prevention of graft versus host disease is done with irradiation (to damage the DNA of donor lymphocytes) of all transfused blood components. she is made
- If the recipient is immunocompromised (eg, patient with congenital immunodeficiency syndrome, hematologic malignancies, hematopoietic stem cell transplantation; neonates)If the donor's blood is obtained from a 1st-degree relative When compatible components are transfused with HLA, with the exception of stem cells
Treatment with corticosteroids and other immunosuppressants, including those used in solid organ transplantation, does not require the use of irradiation.
Circulatory overload associated with transfusion
Although transfusion-associated circulatory overload is underrecognized and underreported, it has recently been identified as the second most common cause of transfusion-related death reported to the FDA (1). The high osmotic load of blood components increases the volume in the intravascular space over hours, which can cause circulatory overload associated with transfusions in susceptible patients (eg, with renal or heart failure). Erythrocytes should be infused slowly. Patients should be observed, and if signs of heart failure (eg, dyspnea, rales) occur, the transfusion should be withheld and treatment for heart failure begun.
Typical treatment is with a diuretic such as furosemide 20 to 40 mg IV. Occasionally, in patients who require a higher volume of plasma infusion to reverse warfarin overload, low-dose furosemide can be applied simultaneously; however, CCP is the first choice for these patients. Patients at high risk for transfusion-associated circulatory overload (eg, with heart failure or severe renal failure) are treated prophylactically with a diuretic (eg, furosemide 20 to 40 mg IV).
Transfusion-related acute lung injury (LPART)
Transfusion-related acute lung injury is an infrequent complication caused by anti-HLA and/or anti-granulocyte antibodies in the donor's plasma that agglutinate and degranulate the recipient's granulocytes within the lung. Acute respiratory symptoms develop, and the chest radiograph has a characteristic pattern of noncardiogenic pulmonary edema. This complication is the second most common cause of transfusion-related death. The incidence is 1 in 5,000 to 1 in 10,000, but many cases are mild. Transfusion-related mild to moderate acute lung injury is likely to go unnoticed. General supportive therapy typically causes recovery without long-lasting sequelae. Diuretics should be avoided. Using blood donated by men reduces the risk of this reaction. Cases must be reported to the blood transfusion service of the hospital where the transfusion is being carried out or to the blood bank.
allergic reactions
Allergic reactions to an unknown component in the donor's blood are common, almost always due to allergens in the donor's plasma or, less frequently, antibodies from an allergic donor. These reactions are usually mild and include hives, swelling, occasional dizziness, and headache during or immediately after the transfusion. Simultaneous fever is common. Less frequently, dyspnea, wheezing, and incontinence may occur, indicating generalized smooth muscle spasm. Anaphylaxis rarely occurs, especially in IgA-deficient recipients.
In a patient with a history of allergies or an allergic reaction to a transfusion, an antihistamine may be given prophylactically just before or at the beginning of the transfusion (eg, 50 mg of diphenhydramine orally or IV). NOTE: Drugs should never be mixed with blood.
If an allergic reaction occurs, the transfusion is stopped. An antihistamine (eg, 50 mg of IV diphenhydramine) usually controls mild hives and pruritus, and the transfusion can be resumed. However, a moderate allergic reaction (generalized urticaria or mild bronchospasm) also requires hydrocortisone (100 to 200 mg IV) and a severe anaphylactic reaction requires additional treatment with 0.5 mL of 1:1000 epinephrine solution subcutaneously and saline at 0.9% IV together with investigation by the blood bank. Another transfusion should not take place until the investigation is complete.
Patients with severe IgA deficiency require transfusion of washed red blood cells, washed platelets, and plasma from the IgA-deficient donor.
Changed oxygen affinity
Blood stored for > 7 days has decreased erythrocyte 2,3-diphosphoglycerate (DPG), and 2,3-DPG is absent after > 10 days. This absence results in a higher affinity for oxygen and slower release of oxygen to the tissues. There is little evidence that 2,3-DPG deficiency is clinically significant, except in exchange transfusions in neonates, in sickle cell disease patients with acute chest pain or stroke, and in some patients with severe heart failure. After erythrocyte transfusion, 2,3-DPG regenerates within 12 to 24 h.
Delayed hemolytic transfusion reaction
Occasionally, a patient sensitized to an erythrocyte antigen has very low antibody levels and negative pre-transfusion tests. After erythrocyte transfusion with this antigen, a primary or anamnestic response may occur (usually within 1 to 4 weeks) and cause a delayed hemolytic reaction. A delayed hemolytic transfusion reaction is usually not as catastrophic as an acute hemolytic transfusion reaction. Patients may be asymptomatic or have a mild fever. Rarely, there are severe symptoms. Often, only destruction of the transfused erythrocytes (with the antigen) occurs, resulting in a fall in hematocrit and a slight increase in lactic dehydrogenase and bilirubin, with a positive direct antiglobulin test. As a delayed hemolytic transfusion reaction is almost always mild and self-limiting, it is often not identified and the clinical clue may be an unexplained drop in hemoglobin to the pre-transfusion level occurring 1 or 2 weeks post-transfusion. Severe reactions are treated similarly to acute reactions.
Infectious diseases
Bacterial contamination of packed red cells occurs rarely, perhaps because of poor aseptic technique during collection or transient bacteremia from the asymptomatic donor. Cooling the erythrocytes usually limits bacterial growth, except for cryophilic microorganisms such as Yersinia, which can produce dangerous levels of endotoxin.
All packed red blood cell bags are examined for bacterial growth prior to release, which is indicated by a change in color. As platelet concentrates are stored at room temperature, they have the greatest potential for bacterial growth and endotoxin production if contaminated. To minimize growth, storage is limited to 5 days. The risk of bacterial contamination of platelets is 1:2,500. In this way, platelets are routinely tested for bacteria.
Rarely, syphilis is transmitted in fresh blood or platelets. Blood storage ≥ 96 h at 4 to 10°C kills the spirochete. Although US federal regulations require blood donor serological testing for syphilis, infected donors are seronegative at the onset of the disease. Recipients of infected units may develop characteristic secondary rashes.
Hepatitis can occur after transfusion of any blood product. The risk has been reduced by viral inactivation by heat treatment of serum albumin and plasma proteins and by the use of concentrated recombinant factors. Hepatitis testing is required for all blood donors (see table Infectious Disease Transmission Tests). The estimated risk of transmission of hepatitis B is 1:1 million; of hepatitis C < 1:2 million. Since its transient viremic phase and concomitant clinical illness are likely to preclude blood donation, hepatitis A (infectious hepatitis) is not a significant cause of transfusion-associated hepatitis.
HIV infection in the US is almost entirely HIV-1, although HIV-2 is also a concern. Testing for antibodies in both diseases is required. Nucleic acid tests for HIV-1 antigen, as well as p24 antigen in HIV-1, are also needed. In addition, blood donors are asked about behaviors that could put them at high risk for HIV infection. HIV-0 has not been identified among blood donors. The estimated risk of HIV transmission by transfusion is 1:1,500,000 to 2,000,000.
Cytomegalovirus (CMV) can be transmitted by leukocytes in transfused blood. It is not transmitted by fresh frozen plasma. Because CMV does not cause disease in immunocompetent recipients, routine antibody testing of donor blood is not required. However, CMV can cause serious or fatal disease in immunocompromised patients, who must receive CMV-negative blood products from donors with negative serology for the virus or from blood with leukodepletion by leukocyte filters.
Human T-cell lymphotropic virus 1 (HTLV-1), which can cause T-cell lymphoma-leukemia in adults and HTLV-1-associated myelopathy/tropical spastic paraparesis, promotes post-transfusional seroconversion in some recipients. All blood donors are tested for antibodies to HTLV-1 and -HTLV-2. The estimated risk of false-negative blood donor test results is 1:641,000.
Creutzfeldt-Jakob disease has never been recorded as transfusion-transmitted, but current practice precludes donation from a person who has received human-derived growth hormone or dura mater transplant, or to a family member with this disease. . Variant Creutzfeldt-Jakob disease (vCJD, or mad cow disease) is not transmitted by blood transfusion. However, donors who have stayed for a significant amount of time in the UK and other parts of Europe are permanently refused donation (see table Some Reasons for Delaying or Denying Blood Donation).
Malaria is easily transmitted through infected erythrocytes. Many donors are not aware that they have had malaria, which can be latent and transmissible for 10 to 15 years. Storage does not make blood safe. Prospective donors should be asked about malaria or if they have been to a region where malaria is prevalent. Donors who have been diagnosed with malaria or are immigrants, refugees, or citizens of countries where malaria is considered endemic are refused for 3 years; travelers from endemic countries are refused for 1 year.
Babesiosis, Chagas disease, and West Nile virus are rarely transmitted by transfusion.
Transmission of Zika virus infection by blood products has been reported in Brazil. Therefore, the FDA mandated testing for Zika virus in the US and its territories. Instead of testing for Zika, approved pathogen reduction technologies for platelets and plasma could also be used; however, its use is currently very restricted, and this technology is not yet available for red blood cells.
post-transfusional purpura
Post-transfusional purpura is a very rare complication in which the platelet count drops rapidly 4 to 14 days after packed red blood cell transfusion, causing moderate to severe thrombocytopenia. Almost all patients are multiparous who normally received blood transfusions during a surgical procedure. The exact etiology is unclear. However, the most accepted hypothesis is that a patient who is negative for human platelet antigen 1a (HPA1a) produces alloantibodies because of exposure to the fetal HPA1a antigen during pregnancy. As stored erythrocytes contain platelet microparticles and as the majority of donors (99%) are HPA1a positive, platelet microparticles from donor blood can trigger an antibody response in previously sensitized patients (anamnestic response). As these platelet microparticles bind to the receptor platelets (and therefore coat them with the HPA1a antigen), the alloantibodies destroy the receptor platelets, causing thrombocytopenia. The disorder spontaneously resolves as the antigen-coated platelets are destroyed.
Patients present with purpura along with moderate to severe bleeding—usually at the surgical site. Platelet and red blood cell transfusions worsen the condition.
The differential diagnosis is usually heparin-induced thrombocytopenia (IPT), although IPT is not associated with bleeding. The diagnosis is made by the identification of antibodies against HPA1a in the patient's plasma and the absence of the corresponding antigen in the patient's platelets.
Treatment is with high doses of IV immunoglobulins (1 to 2 g/kg) and avoiding new transfusions of platelets or red blood cells. Plasmapheresis may be considered in severe cases, and for patients with severe bleeding, platelet transfusions from HPA1a-negative donors may be given, if available.
Complications of massive transfusion
Massive transfusion is the transfusion of a volume of blood greater than or equal to one blood volume in 24 hours (eg, 10 units in an adult weighing 70 kg). When a patient receives conventional fluid replacement with red blood cells (colloid) and crystalloids (saline or lactated Ringer's) in such a high volume, the clotting factors and platelets in the plasma are diluted, causing coagulopathy (dilutional coagulopathy). This coagulopathy worsens consumption coagulopathy from the severe trauma itself (resulting from extensive activation of the coagulation cascade) and leads to a lethal triad of acidosis, hypothermia, and bleeding.
Recently, massive transfusion protocols have been developed in which platelets and fresh frozen plasma are given earlier in resuscitation, before coagulopathy develops, rather than trying to "recover lost ground." These protocols have been shown to decrease the mortality rate, although the optimal proportions of red blood cells, plasma, and platelets are still being investigated. A recent study showed no significant difference in mortality between administering 1 unit of plasma and platelet concentrate every 2 units of packed red blood cells (1:1:2) versus administering 1 unit of plasma and platelet concentrate every 1 unit of packed red blood cells [1:1:1 (2)].
Hypothermia resulting from the rapid transfusion of large amounts of cooled blood can cause arrhythmias or cardiac arrest. Hypothermia is avoided by using a heat exchange IV device that gently warms the blood. Other types of blood heating (eg, microwaves) are contraindicated because of potential erythrocyte damage and hemolysis.
The toxic effects of citrate and potassium are generally not of concern, even with massive transfusion; however, the toxic effects of both can be potentiated in case of hypothermia. Patients with hepatic impairment may have difficulty metabolizing citrate. Hypocalcemia may occur, but treatment is rarely needed (with 10 mL of 10% calcium gluconate solution IV diluted in 100 mLD5W, given over 10 minutes). Patients with renal insufficiency may have increased potassium levels if they are transfused with blood stored for > 1 week (potassium accumulation is usually negligible in blood stored for< 1 semana). A hemólise mecânica durante a transfusão pode aumentar os níveis de potássio. Pode ocorrer hipopotassemia cerca de 24 h após a transfusão de eritrócitos senescentes (> 3 weeks), which absorb potassium.
General references
- 1. FDA: Fatalities reported to FDA following blood collection and transfusion: Annual Summary for Fiscal Year 2018. Silver Spring, MD, US Food and Drug Administration, 2018.2. Holcomb JB, Tilley BC, Baraniuk S, et al: Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 313(5):471–482, 2015. doi:10.1001/jama.2015.12
Diseases that affect the blood
Some diseases directly affect blood cells, triggering a series of unpleasant and even fatal consequences. Here are some of these diseases:
Anemia: when there is a reduction in the amount of hemoglobin in the blood. Anemia can be triggered, for example, by a lack of iron in the diet and bleeding. The anemic individual presents, among other symptoms, weakness, tiredness, shortness of breath and dizziness.
Sickle Cell Anemia: when there is a change in the red blood cells, which have a sickle shape. This change can trigger the formation of clots that lead to the obstruction of blood vessels, which can cause damage to certain organs. In sickle cell anemia, the individual may experience pain and fatigue.
Hemophilia: is a genetic problem, linked to the X chromosome, which causes changes in blood clotting. This means that people with this condition may experience excessive bleeding from an injury. The treatment of hemophilia is usually based on replacing the clotting factor that is not present in the patient.
Leukemia: is a type of cancer that affects white blood cells and is characterized by the production of abnormal cells. There are, according to the National Cancer Institute (Inca), more than 12 different types of leukemia. The treatment of anemia is varied and may include chemotherapy, radiation therapy and also bone marrow transplantation.
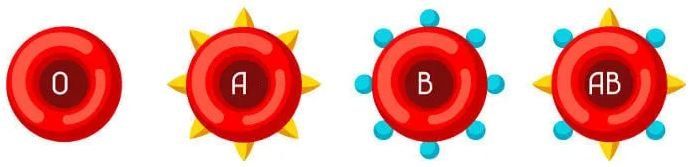
blood types
We know that in the human species we have four different blood types: type A, type B, type AB and type O.
People with blood type A have agglutinogen A in the red blood cells, while people with blood type B have agglutinogen B. People with blood type AB have agglutinogen A. and B, while in people with blood type O, no agglutinogen is observed in red blood cells.